The Orbital Diagram Shows the Valence Electrons of Sulfur
The orbital diagram shows the valence electrons of sulfur which has 16 electrons if the electrons were added to the atom one at a time which would be the last electron to occupy an orbital Answers. Again the electron configuration of sulfur excited state is S 16 1s 2 2s 2 2p 6 3s 1 3p x1 3p y1 3p z1 3d xy1 3d yz1.

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2
For example He 2s22p2 would be entered as.

. From this configuration it has been seen that the last electron 16th electron occupies a 3p orbital 3p⁴ orbital with a downward symbol. The arrows represent the 16 electrons of the sulfur atom and the directions represent their spins. The Orbital Diagram for Sulfur.
Correct answer - The orbital diagram shows the valence electrons of sulfur which has 16 electrons. Therefore the first two electrons will go in 1s orbital the next. The p orbital can hold up to six electronsShow the orbital-filling diagram for S sulfur.
Figure 1 What is the element. Sulfurs electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. The aufbau principle the Pauli exclusion principle and Hunds rule.
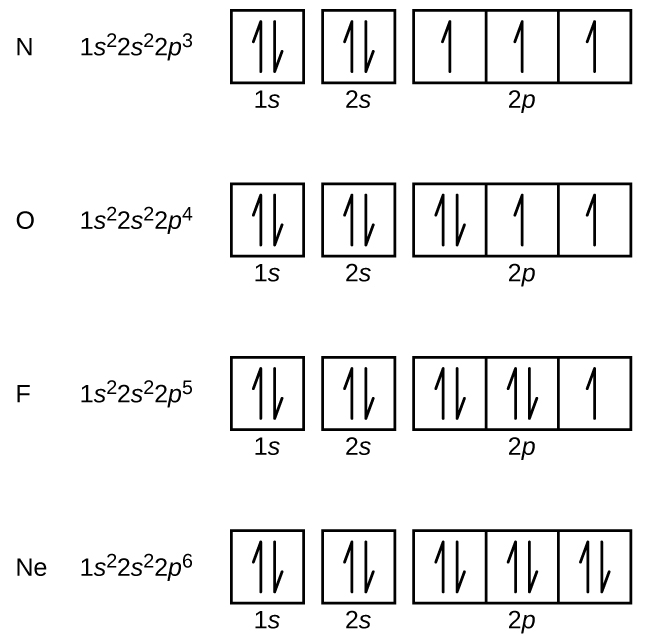
The number of unpaired electrons in the last orbit of an element is the valency of that element. Up to the 2sp electrons is the noble gas configuration of neon. X 1s 2s 2p 3s 3p 11 12 TUTTI I 11111 1s 2s 2p 3s Зр TJ 1111111.
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. In this case sulfur has six unpaired electrons. The boxes represent sulfurs orbitals.
What is the Orbital diagram for Sulfur. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each.
Express your answer in condensed form. To see this video other videos chemistry education text and practice problems visit my website. Chemistry questions and answers.
Be sure to follow the three orbital filling rules. 13 141 1s 2s 2p 3s Зр 11 11 111111. There is a shorthand way of writing electron configurations.
Sulfur has a total of 16 electrons and one box can hold up to two electrons. What is the electron configuration of an atom of this element. Well we use the aufbau principle and for sulfur Z The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each.
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Express your answer as a chemical symbol. Since 1s can only hold two electrons the next 2.
Orbital diagram-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom it shows the electrons in the form of arrows also indicates the spin of electronsElectron configuration- Electron configuration is the arrangement of electrons in atomic orbitalsIt shows the electrons in numbers It doesnt show the details on the spin of. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. An sulfur atom S has 16 electrons means having an electron configuration as follows.
1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes. The next six electrons will go in the 2p orbital. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom.
The orbital diagram of Sulfur contains 1s orbital 2s orbital 2p orbital 3s orbital and 3p orbital. For this the valency valence of sulfur is 4. If the electrons were added to the atom one at a tim Subjects.
For example the electron configuration for sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. When we write the configuration well put all 16 electrons in orbitals around the nucleus of the Sulfur atom. SulphurSulfur S has an atomic mass of Find out about its chemical and physical properties states energy electrons oxidation and more.
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. The orbital diagram that follows shows the valence electrons for a 2 ion of an element. Iron has 8 valence electrons with 2 electrons in the 4s subshell and 6 electrons in the 3d subshell.
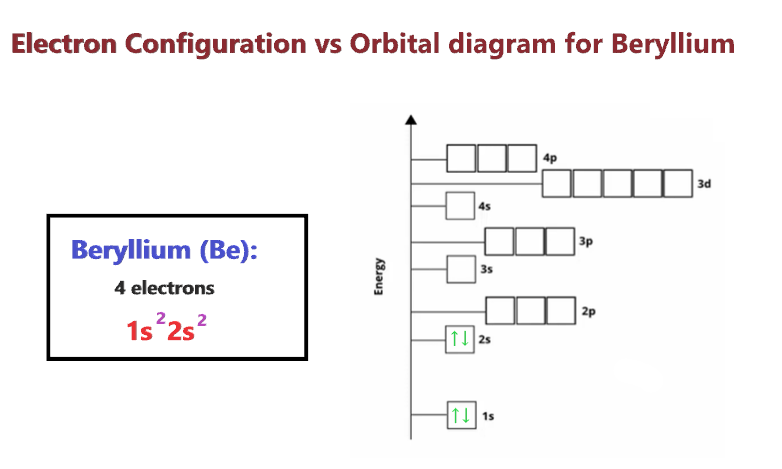
The number of valence electrons impacts on their chemical properties and the specific ordering and properties of the orbitals are important in physics so many students have to get to grips with the basics. Choose the correct orbital diagram for the ground-state electron arrangement for sulfur. Arrows within boxes Draw an orbital diagram for beryllium Z4.
The orbital diagram shows the valence electrons of sulfur which has 16 electrons. Show the orbital-filling diagram for bromineStatus. In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom there are 16 electrons.
Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. 1s² 2s² 2p⁶ 3s² 3p⁴. Boxes or lines represent each orbital.
If the electrons were added to the atom one a. Orbital Box Diagram For Sulfur. Or we can write with the noble gas symbol.
The valence electron configurations for all of the elements are in the periodic table below. 2 8 6 Orbitals.
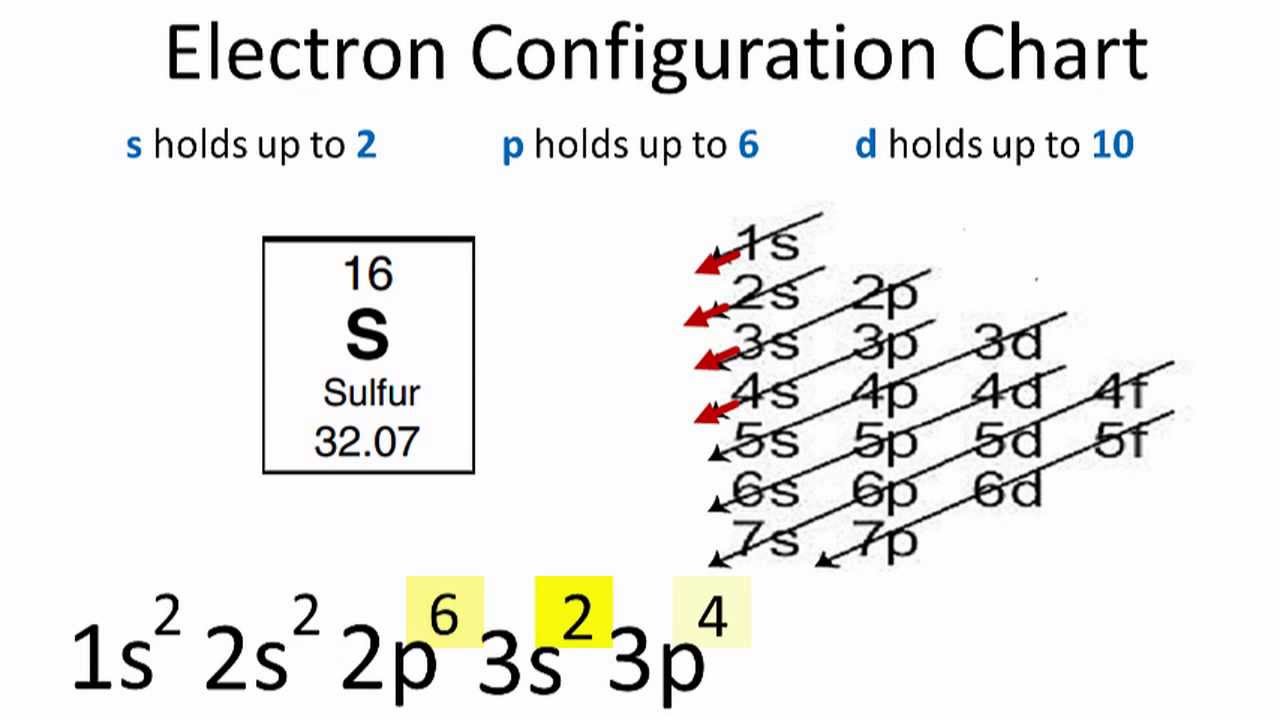
Electron Configuration For Sulfur S
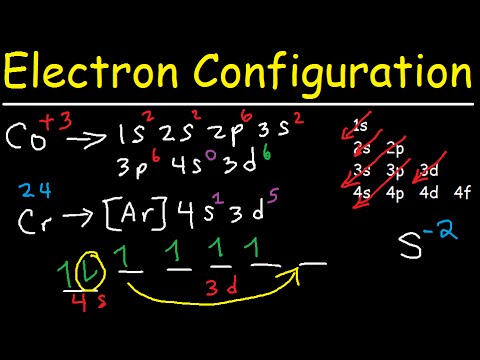
Electron Configuration Quick Review Youtube
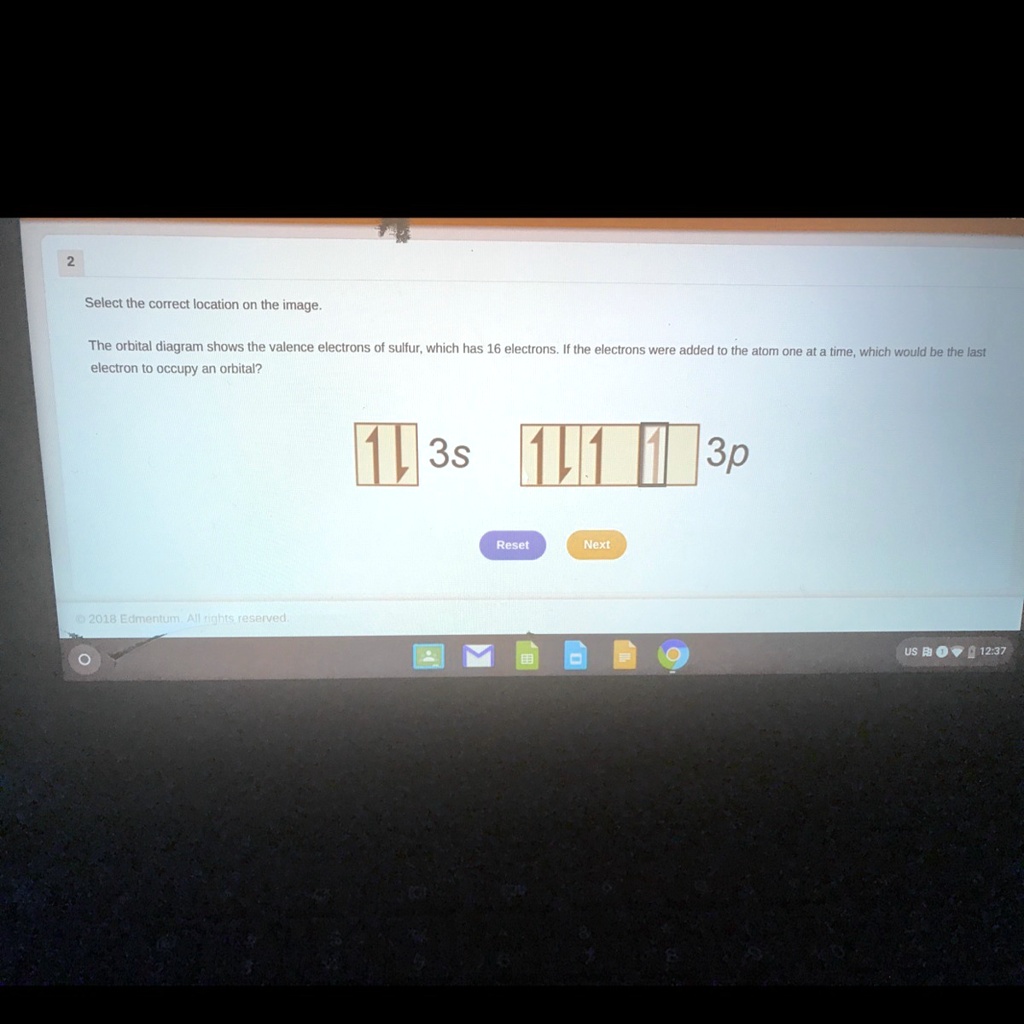
Solved Someone Please Help Select The Correct Location On The Image The Orbital Diagram Shows The Valence Electrons Of Sulfur Which Has 16 Electrons If The Electrons Were Added T0 The Atom One

Sulfur Orbital Diagram Electron Configuration And Valence Electrons

The Orbital Diagram Shows The Valence Electrons Of Sulfur Which Has 16 Electrons If The Electrons Brainly Com

Orbital Diagrams Overview Examples Expii
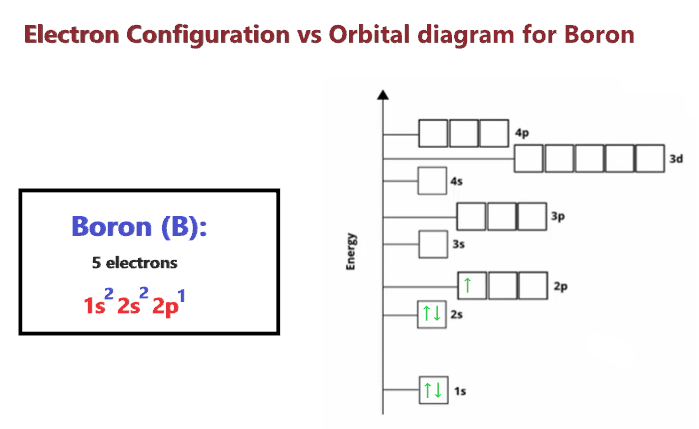
Boron Orbital Diagram Electron Configuration And Valence Electrons

Orbital Diagrams Overview Examples Expii

Electron Configuration For Calcium Ca

Sulfur Orbital Diagram Electron Configuration And Valence Electrons

Sulfur Orbital Diagram Electron Configuration And Valence Electrons

Orbital Diagrams Overview Examples Expii

How To Draw Orbital Diagrams For Any Atom Orbital Notation

S 2 Electron Configuration Sulfide Ion Youtube
![]()
Silicon Orbital Diagram Electron Configuration And Valence Electrons
Beryllium Orbital Diagram Electron Configuration And Valence Electrons
3 1 Electron Configurations Chemistry Libretexts
Sulfur Orbital Diagram Electron Configuration And Valence Electrons

Comments
Post a Comment